A difference of four units, from six to two, means that the ...
Próximas questões
Com base no mesmo assunto
Ano: 2014
Banca:
IDECAN
Órgão:
CNEN
Provas:
IDECAN - 2014 - CNEN - Analista de Tecnologia da Informação/ Governança e Gestão
|
IDECAN - 2014 - CNEN - Enfermeiro |
Q514978
Inglês
Texto associado
Acid rain and… the facts
www.acidrain.org.ca / Oxford Children´s Encyclopedia
What causes acid rain?
Acid rain is caused by air pollution. When fossil fuels such as coal and oil are burned, two gases, sulphur dioxide and nitrogen oxide, are released into the atmosphere. These two pollutants eventually react with the moisture in the air. When this polluted mixture falls onto the ground, it is called acid rain.
Rain measuring between 0 and 5 on the pH scale, is acidic therefore called ACID RAIN.
Acid rain is harmful to the environment. It is hardto control because it may be blown by the wind, falling thousands of kilometers from where it was first formed. For example, much of the acid rain in Canada is caused by smoke from factories and power-stations in the USA. The acid rain in Scandinavia may come from Britain.
What are the effects of acid rain?
Acid rain has many different effects. It has killedfish in the lakes of North America, Scandinavia, Scotland, and Wales. Vast areas of forest in northern and central Europeare dying because of it, while in many European cities statues and stone buildings are being eaten away by the acid. Acid rain corrodes metalwork such as steel bridges and railings; it also attacks some types of concrete. Even the water thatwe drink is slowly being polluted by acid rain.
What are the effects on trees and soil?
One of the most serious impacts of acid precipitation is on forests and soils. Great damage is done when sulphuric acid falls onto the earth as rain. Nutrients present in the soils are washed away. Aluminium also present in the soils is freed and this toxic element can be absorbed by the rootsof trees. Thus, the trees starve to death because they have been deprived of their vital nutrients such as calcium and magnesium.
Acid rain is one of the most serious environmental problems of our time. It is a global problems that is gradually affecting our world.
How does acid rain effect lakes?
Lakes that have been acidified cannot support the same variety of life as healthy lakes. As a lake becomes more acidic, various types of fish disappear. Other effects of acidified lakes on fish include: decreased growth, inability to regulate their own body chemistry, reduced egg deposition, deformities in young fish and increased susceptibility to naturally occurring diseases.
Clean rain usually has a pH of 5.6. It is slightly acidic because of carbon dioxide which is naturally present in the atmosphere. Vinegar, by comparison, is very acidic and has a pH of 3.
What is pH?
This is a measure of how acidic or alkaline a substance is. (See a pH scale below).
The initials pH stand for Potential of Hydrogen. Acids have pH values under 7, and alkalis have pHvalues over 7. If a substance has a pH value of 7. It is neutral-neither acidic or alkaline.
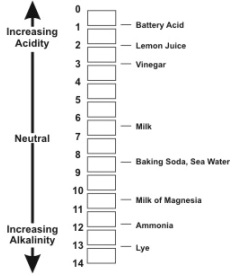
Because the pH scale is logarithmic, a difference of one pH unit represents a tenfold, or ten times change. For example, the acidity of a sample with a pH of 5 is ten timesgreater than that of a sample with a pH of 6. A difference of 2 units, from 6 to 4, would mean that the acidity in one hundred times greater, and so on.
(Reinildes Dias. Reading Critically in English, 3 rd ed. UFMG 2002. Adaptado.)
Acid rain and… the facts
www.acidrain.org.ca / Oxford Children´s Encyclopedia
What causes acid rain?
Acid rain is caused by air pollution. When fossil fuels such as coal and oil are burned, two gases, sulphur dioxide and nitrogen oxide, are released into the atmosphere. These two pollutants eventually react with the moisture in the air. When this polluted mixture falls onto the ground, it is called acid rain.
Rain measuring between 0 and 5 on the pH scale, is acidic therefore called ACID RAIN.
Acid rain is harmful to the environment. It is hardto control because it may be blown by the wind, falling thousands of kilometers from where it was first formed. For example, much of the acid rain in Canada is caused by smoke from factories and power-stations in the USA. The acid rain in Scandinavia may come from Britain.
What are the effects of acid rain?
Acid rain has many different effects. It has killedfish in the lakes of North America, Scandinavia, Scotland, and Wales. Vast areas of forest in northern and central Europeare dying because of it, while in many European cities statues and stone buildings are being eaten away by the acid. Acid rain corrodes metalwork such as steel bridges and railings; it also attacks some types of concrete. Even the water thatwe drink is slowly being polluted by acid rain.
What are the effects on trees and soil?
One of the most serious impacts of acid precipitation is on forests and soils. Great damage is done when sulphuric acid falls onto the earth as rain. Nutrients present in the soils are washed away. Aluminium also present in the soils is freed and this toxic element can be absorbed by the rootsof trees. Thus, the trees starve to death because they have been deprived of their vital nutrients such as calcium and magnesium.
Acid rain is one of the most serious environmental problems of our time. It is a global problems that is gradually affecting our world.
How does acid rain effect lakes?
Lakes that have been acidified cannot support the same variety of life as healthy lakes. As a lake becomes more acidic, various types of fish disappear. Other effects of acidified lakes on fish include: decreased growth, inability to regulate their own body chemistry, reduced egg deposition, deformities in young fish and increased susceptibility to naturally occurring diseases.
Clean rain usually has a pH of 5.6. It is slightly acidic because of carbon dioxide which is naturally present in the atmosphere. Vinegar, by comparison, is very acidic and has a pH of 3.
What is pH?
This is a measure of how acidic or alkaline a substance is. (See a pH scale below).
The initials pH stand for Potential of Hydrogen. Acids have pH values under 7, and alkalis have pHvalues over 7. If a substance has a pH value of 7. It is neutral-neither acidic or alkaline.

Because the pH scale is logarithmic, a difference of one pH unit represents a tenfold, or ten times change. For example, the acidity of a sample with a pH of 5 is ten timesgreater than that of a sample with a pH of 6. A difference of 2 units, from 6 to 4, would mean that the acidity in one hundred times greater, and so on.
(Reinildes Dias. Reading Critically in English, 3 rd ed. UFMG 2002. Adaptado.)
A difference of four units, from six to two, means that the acidity is