Questões de Vestibular Sobre inglês
Foram encontradas 5.992 questões
1 Before Hurricane Sandy tore through New York and New Jersey, it stopped in Florida. Huge waves covered beaches, swept over Fort Lauderdale's concrete sea wall and spilled onto A1A, Florida's coastal highway. A month later another series of violent storms hit south Florida, severely eroding Fort Lauderdale's beaches and a section of A1A. Workers are building a new sea wall, mending the highway and adding a couple of pedestrian bridges. Beach erosion forced Fort Lauderdale to buy sand from an inland mine in central Florida; the mine's soft, white sand stands out against the darker, grittier native variety.
2 Hurricanes and storms are nothing new for Florida. But as the oceans warm, hurricanes are growing more intense. To make matters worse, this is happening against a backdrop of sharply rising sea levels, turning what has been a seasonal annoyance into an existential threat.
3 For around 2,000 years sea levels remained relatively constant. Between 1880 and 2011, however, they rose by an average of 0.07 inches (1.8mm) a year, and between 1993 and 2011 the average was between 0.11 and 0.13 inches a year. In 2007 the Intergovernmental Panel on Climate Change (IPCC) forecast that seas could rise by as much as 23 inches by 2100, though since then many scientists have called that forecast conservative. Seas are also expected to warm up, which may make hurricanes and tropical storms more intense.
4 Even as seas have risen over the past century, Americans have rushed to build homes near the beach. Storms that lash the modern American coastline cause more economic damage than their predecessors because there is more to destroy. The Great Miami Hurricane of 1926, a Category 4 storm, caused $1 billion-worth of damage in current dollars. Were it to strike today the insured losses would be $125 billion, reckons Air Worldwide, a catastrophe-modelling firm. In 1992 Hurricane Andrew, a Category 5 storm, caused $23 billion in damage; today it would be twice that
5 Most Floridians live in coastal counties. Buildings cluster on low ground; more people than in any other state live on land less than four feet (1.2 metres) above the high-tide line. Florida's limestone bedrock makes it easy for salt water from surging seas to contaminate its freshwater aquifers. And it relies heavily on canals for flood control, which a sea-level rise of just six inches would devastate.
Adapted from The Economist, June 15th , 2013
June 26, 2014
By Amy Graff

Reading Go Dog Go to your 6 month old might seem like wasted time because she’s more likely to eat the book than help you turn the pages, but a statement released by the American Academy of Pediatrics (AAP) this week says reading in the early years is essential. Reading out loud gets parents talking to their babies and the sound of an adult’s voice stimulates that tiny yet rapidly growing brain. In the statement, the academy advises pediatricians to tell parents to read books to their children from birth.
Reading regularly with young children stimulates optimal patterns of brain development and strengthens parent-child relationships at a critical time in child development, which, in turn, builds language, literacy, and social-emotional skills that last a lifetime. Research shows that a child’s brain develops faster between 0 and 3 than at any other time in life, making the early years a critical time for babies to hear rich oral language. The more words children hear directed at them by parents and caregivers, the more they learn.
While many babies are read Goodnight Moon and The Very Hungry Caterpillar every night before bed, others never get a chance to “pat the bunny.” Studies reveal that children from low-income, less-educated families have significantly fewer books than their more affluent peers. By age 4, children in poverty hear 30 million fewer words than those in higher-income households. These dramatic gaps result in significant learning disadvantages that persist into adulthood. The AAP hopes the new guidelines will encourage all parents to start reading from day one.
Research shows that when pediatricians talk with parents about reading, moms and dads are more likely to fill their home with books and read. Also, to help get more parents reading, the AAP is partnering with organizations such as Scholastic and Too Small to Fail to help get reading materials to new families who need books the most.
This is the first time the AAP has made a recommendation on children’s literary education and it seems the timing might be just right as more and more parents are leaning on screens and electronic gadget to occupy their babies. “The reality of today’s world is that we’re competing with portable digital media,” Dr. Alanna Levine, a pediatrician in Orangeburg, N.Y., told The New York Times. “So you really want to arm parents with tools and rationale behind it about why it’s important to stick to the basics of things like books.”
(http://blog.seattlepi.com. Adaptado.)
June 26, 2014
By Amy Graff

Reading Go Dog Go to your 6 month old might seem like wasted time because she’s more likely to eat the book than help you turn the pages, but a statement released by the American Academy of Pediatrics (AAP) this week says reading in the early years is essential. Reading out loud gets parents talking to their babies and the sound of an adult’s voice stimulates that tiny yet rapidly growing brain. In the statement, the academy advises pediatricians to tell parents to read books to their children from birth.
Reading regularly with young children stimulates optimal patterns of brain development and strengthens parent-child relationships at a critical time in child development, which, in turn, builds language, literacy, and social-emotional skills that last a lifetime. Research shows that a child’s brain develops faster between 0 and 3 than at any other time in life, making the early years a critical time for babies to hear rich oral language. The more words children hear directed at them by parents and caregivers, the more they learn.
While many babies are read Goodnight Moon and The Very Hungry Caterpillar every night before bed, others never get a chance to “pat the bunny.” Studies reveal that children from low-income, less-educated families have significantly fewer books than their more affluent peers. By age 4, children in poverty hear 30 million fewer words than those in higher-income households. These dramatic gaps result in significant learning disadvantages that persist into adulthood. The AAP hopes the new guidelines will encourage all parents to start reading from day one.
Research shows that when pediatricians talk with parents about reading, moms and dads are more likely to fill their home with books and read. Also, to help get more parents reading, the AAP is partnering with organizations such as Scholastic and Too Small to Fail to help get reading materials to new families who need books the most.
This is the first time the AAP has made a recommendation on children’s literary education and it seems the timing might be just right as more and more parents are leaning on screens and electronic gadget to occupy their babies. “The reality of today’s world is that we’re competing with portable digital media,” Dr. Alanna Levine, a pediatrician in Orangeburg, N.Y., told The New York Times. “So you really want to arm parents with tools and rationale behind it about why it’s important to stick to the basics of things like books.”
(http://blog.seattlepi.com. Adaptado.)
June 26, 2014
By Amy Graff

Reading Go Dog Go to your 6 month old might seem like wasted time because she’s more likely to eat the book than help you turn the pages, but a statement released by the American Academy of Pediatrics (AAP) this week says reading in the early years is essential. Reading out loud gets parents talking to their babies and the sound of an adult’s voice stimulates that tiny yet rapidly growing brain. In the statement, the academy advises pediatricians to tell parents to read books to their children from birth.
Reading regularly with young children stimulates optimal patterns of brain development and strengthens parent-child relationships at a critical time in child development, which, in turn, builds language, literacy, and social-emotional skills that last a lifetime. Research shows that a child’s brain develops faster between 0 and 3 than at any other time in life, making the early years a critical time for babies to hear rich oral language. The more words children hear directed at them by parents and caregivers, the more they learn.
While many babies are read Goodnight Moon and The Very Hungry Caterpillar every night before bed, others never get a chance to “pat the bunny.” Studies reveal that children from low-income, less-educated families have significantly fewer books than their more affluent peers. By age 4, children in poverty hear 30 million fewer words than those in higher-income households. These dramatic gaps result in significant learning disadvantages that persist into adulthood. The AAP hopes the new guidelines will encourage all parents to start reading from day one.
Research shows that when pediatricians talk with parents about reading, moms and dads are more likely to fill their home with books and read. Also, to help get more parents reading, the AAP is partnering with organizations such as Scholastic and Too Small to Fail to help get reading materials to new families who need books the most.
This is the first time the AAP has made a recommendation on children’s literary education and it seems the timing might be just right as more and more parents are leaning on screens and electronic gadget to occupy their babies. “The reality of today’s world is that we’re competing with portable digital media,” Dr. Alanna Levine, a pediatrician in Orangeburg, N.Y., told The New York Times. “So you really want to arm parents with tools and rationale behind it about why it’s important to stick to the basics of things like books.”
(http://blog.seattlepi.com. Adaptado.)
June 26, 2014
By Amy Graff

Reading Go Dog Go to your 6 month old might seem like wasted time because she’s more likely to eat the book than help you turn the pages, but a statement released by the American Academy of Pediatrics (AAP) this week says reading in the early years is essential. Reading out loud gets parents talking to their babies and the sound of an adult’s voice stimulates that tiny yet rapidly growing brain. In the statement, the academy advises pediatricians to tell parents to read books to their children from birth.
Reading regularly with young children stimulates optimal patterns of brain development and strengthens parent-child relationships at a critical time in child development, which, in turn, builds language, literacy, and social-emotional skills that last a lifetime. Research shows that a child’s brain develops faster between 0 and 3 than at any other time in life, making the early years a critical time for babies to hear rich oral language. The more words children hear directed at them by parents and caregivers, the more they learn.
While many babies are read Goodnight Moon and The Very Hungry Caterpillar every night before bed, others never get a chance to “pat the bunny.” Studies reveal that children from low-income, less-educated families have significantly fewer books than their more affluent peers. By age 4, children in poverty hear 30 million fewer words than those in higher-income households. These dramatic gaps result in significant learning disadvantages that persist into adulthood. The AAP hopes the new guidelines will encourage all parents to start reading from day one.
Research shows that when pediatricians talk with parents about reading, moms and dads are more likely to fill their home with books and read. Also, to help get more parents reading, the AAP is partnering with organizations such as Scholastic and Too Small to Fail to help get reading materials to new families who need books the most.
This is the first time the AAP has made a recommendation on children’s literary education and it seems the timing might be just right as more and more parents are leaning on screens and electronic gadget to occupy their babies. “The reality of today’s world is that we’re competing with portable digital media,” Dr. Alanna Levine, a pediatrician in Orangeburg, N.Y., told The New York Times. “So you really want to arm parents with tools and rationale behind it about why it’s important to stick to the basics of things like books.”
(http://blog.seattlepi.com. Adaptado.)
June 26, 2014
By Amy Graff

Reading Go Dog Go to your 6 month old might seem like wasted time because she’s more likely to eat the book than help you turn the pages, but a statement released by the American Academy of Pediatrics (AAP) this week says reading in the early years is essential. Reading out loud gets parents talking to their babies and the sound of an adult’s voice stimulates that tiny yet rapidly growing brain. In the statement, the academy advises pediatricians to tell parents to read books to their children from birth.
Reading regularly with young children stimulates optimal patterns of brain development and strengthens parent-child relationships at a critical time in child development, which, in turn, builds language, literacy, and social-emotional skills that last a lifetime. Research shows that a child’s brain develops faster between 0 and 3 than at any other time in life, making the early years a critical time for babies to hear rich oral language. The more words children hear directed at them by parents and caregivers, the more they learn.
While many babies are read Goodnight Moon and The Very Hungry Caterpillar every night before bed, others never get a chance to “pat the bunny.” Studies reveal that children from low-income, less-educated families have significantly fewer books than their more affluent peers. By age 4, children in poverty hear 30 million fewer words than those in higher-income households. These dramatic gaps result in significant learning disadvantages that persist into adulthood. The AAP hopes the new guidelines will encourage all parents to start reading from day one.
Research shows that when pediatricians talk with parents about reading, moms and dads are more likely to fill their home with books and read. Also, to help get more parents reading, the AAP is partnering with organizations such as Scholastic and Too Small to Fail to help get reading materials to new families who need books the most.
This is the first time the AAP has made a recommendation on children’s literary education and it seems the timing might be just right as more and more parents are leaning on screens and electronic gadget to occupy their babies. “The reality of today’s world is that we’re competing with portable digital media,” Dr. Alanna Levine, a pediatrician in Orangeburg, N.Y., told The New York Times. “So you really want to arm parents with tools and rationale behind it about why it’s important to stick to the basics of things like books.”
(http://blog.seattlepi.com. Adaptado.)
June 26, 2014
By Amy Graff

Reading Go Dog Go to your 6 month old might seem like wasted time because she’s more likely to eat the book than help you turn the pages, but a statement released by the American Academy of Pediatrics (AAP) this week says reading in the early years is essential. Reading out loud gets parents talking to their babies and the sound of an adult’s voice stimulates that tiny yet rapidly growing brain. In the statement, the academy advises pediatricians to tell parents to read books to their children from birth.
Reading regularly with young children stimulates optimal patterns of brain development and strengthens parent-child relationships at a critical time in child development, which, in turn, builds language, literacy, and social-emotional skills that last a lifetime. Research shows that a child’s brain develops faster between 0 and 3 than at any other time in life, making the early years a critical time for babies to hear rich oral language. The more words children hear directed at them by parents and caregivers, the more they learn.
While many babies are read Goodnight Moon and The Very Hungry Caterpillar every night before bed, others never get a chance to “pat the bunny.” Studies reveal that children from low-income, less-educated families have significantly fewer books than their more affluent peers. By age 4, children in poverty hear 30 million fewer words than those in higher-income households. These dramatic gaps result in significant learning disadvantages that persist into adulthood. The AAP hopes the new guidelines will encourage all parents to start reading from day one.
Research shows that when pediatricians talk with parents about reading, moms and dads are more likely to fill their home with books and read. Also, to help get more parents reading, the AAP is partnering with organizations such as Scholastic and Too Small to Fail to help get reading materials to new families who need books the most.
This is the first time the AAP has made a recommendation on children’s literary education and it seems the timing might be just right as more and more parents are leaning on screens and electronic gadget to occupy their babies. “The reality of today’s world is that we’re competing with portable digital media,” Dr. Alanna Levine, a pediatrician in Orangeburg, N.Y., told The New York Times. “So you really want to arm parents with tools and rationale behind it about why it’s important to stick to the basics of things like books.”
(http://blog.seattlepi.com. Adaptado.)
June 26, 2014
By Amy Graff

Reading Go Dog Go to your 6 month old might seem like wasted time because she’s more likely to eat the book than help you turn the pages, but a statement released by the American Academy of Pediatrics (AAP) this week says reading in the early years is essential. Reading out loud gets parents talking to their babies and the sound of an adult’s voice stimulates that tiny yet rapidly growing brain. In the statement, the academy advises pediatricians to tell parents to read books to their children from birth.
Reading regularly with young children stimulates optimal patterns of brain development and strengthens parent-child relationships at a critical time in child development, which, in turn, builds language, literacy, and social-emotional skills that last a lifetime. Research shows that a child’s brain develops faster between 0 and 3 than at any other time in life, making the early years a critical time for babies to hear rich oral language. The more words children hear directed at them by parents and caregivers, the more they learn.
While many babies are read Goodnight Moon and The Very Hungry Caterpillar every night before bed, others never get a chance to “pat the bunny.” Studies reveal that children from low-income, less-educated families have significantly fewer books than their more affluent peers. By age 4, children in poverty hear 30 million fewer words than those in higher-income households. These dramatic gaps result in significant learning disadvantages that persist into adulthood. The AAP hopes the new guidelines will encourage all parents to start reading from day one.
Research shows that when pediatricians talk with parents about reading, moms and dads are more likely to fill their home with books and read. Also, to help get more parents reading, the AAP is partnering with organizations such as Scholastic and Too Small to Fail to help get reading materials to new families who need books the most.
This is the first time the AAP has made a recommendation on children’s literary education and it seems the timing might be just right as more and more parents are leaning on screens and electronic gadget to occupy their babies. “The reality of today’s world is that we’re competing with portable digital media,” Dr. Alanna Levine, a pediatrician in Orangeburg, N.Y., told The New York Times. “So you really want to arm parents with tools and rationale behind it about why it’s important to stick to the basics of things like books.”
(http://blog.seattlepi.com. Adaptado.)

No contexto do quadrinho, o termo “can” indica uma ideia de

A expressão “instead of” equivale, em português, a

The boy
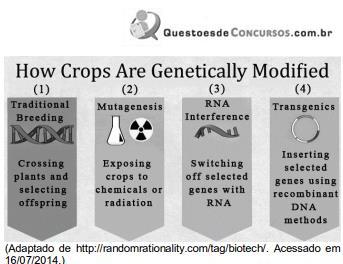
Qual das técnicas descritas no infográfico acima foi utilizada por Gregor Mendel (1822-1884) em seus experimentos?
Some 80 percent of all the planet's earthquakes occur along the rim of the Pacific Ocean, called the "Ring of Fire" because of the preponderance of volcanic activity there. Most earthquakes occur at fault zones, where tectonic plates – giant rock slabs that make up the Earth's upper layer – collide or slide against each other. These impacts are usually gradual and unnoticeable on the surface; however, immense stress can build up between plates. When this stress is released quickly, it sends massive vibrations, called seismic waves, often hundreds of miles through the rock and up to the surface.
(Adaptado de http://environment.nationalgeographic.com/envir onment/natural-disasters/earthquake-profile/. Acessado em 22/06/2014.)
De acordo com o texto,

O cartaz acima critica, de forma irônica,
Dog and cat lovers seem to relish unending debates over which animal is "smarter." Dog owners often cap their arguments with the fact that dogs have the ability to perform tricks, while cat people counter with the claim that their pets are too intelligent to perform on command. In truth, such methods of pet comparison are useless animal-world versions of mixing apples and oranges. Dogs are motivated by a strong need to follow and please their masters in order to receive praise. The solitary cat answers to no one; nevertheless, if trainability may not be the feline's forte, cleverness and adaptability certainly are.
(Adaptado de http://www.animalplanet.com/pets/cat-intelligence.htm. Acessado em 14/06/2014.)
Segundo o texto,
Tyrannosaurus rex was one of the largest meat- eating dinosaurs that ever lived. Fossil evidence shows that T. rex was about 12 meters long and about 4.6 to 6 meters tall. Its robust thighs and long, powerful tail helped it move quickly.
T. rex's serrated, conical teeth were used to pierce and grip flesh, which it then ripped away with its strong neck muscles. Its two-fingered forearms could probably seize prey, but they were too short to reach its mouth.
(Adaptado de http://animals.nationalgeographic.com/animals/ prehistoric/tyrannosaurus-rex/. Acessado em 15/06/2014.)
Segundo o texto,

(Disponível em http://www.lifebuzz.com/funny-texts/#!SsbFU. Acessado em 02/02/2014.)
Depreende-se dessa troca de mensagens que

O texto reproduzido no poster acima corresponde a um verso de uma canção escrita por John Lennon e gravada pela banda The Beatles em 1967. Da leitura desse verso se depreende que viver só é fácil para pessoas

Para o menino do cartum é surpreendente que seus avós

O personagem do cartum