Questões de Inglês - Advérbios e conjunções | Adverbs and conjunctions para Concurso
Foram encontradas 517 questões
Julgue o item subsequente.
Correlative conjunctions, exemplified by pairs like
“either...or” and “neither...nor,” work together to connect
grammatically parallel elements. The correct usage of
correlative conjunctions ensures a balanced and
harmonious structure within sentences, promoting
effective communication in American English.
Understanding the interplay between these paired
conjunctions facilitates the construction of well-formed
and logically connected expressions in both written and
spoken contexts.


Internet: <www.britannica.com> (with adaptations).
The adverb “bloodily” (line 18) follows the same spelling rule of the adverbs nicely, simply and easily.

Internet: <www.britannica.com> (with adaptations).
The word “but” in “Bahia was still controlled by forces loyal to Portugal, but on July 2, 1823, Brazilian troops occupied Salvador, and Bahia became a province of the empire.” (lines 14 and 15) can be correctly replaced by the word however.
TEXT II – Tema: Variação linguística no ensino-aprendizagem de inglês
INTERNATIONAL VARIETIES OF ENGLISH
1 The most familiar way to classify the varieties of English around the world was developed by
2 Kachru (1985) and looks like this:
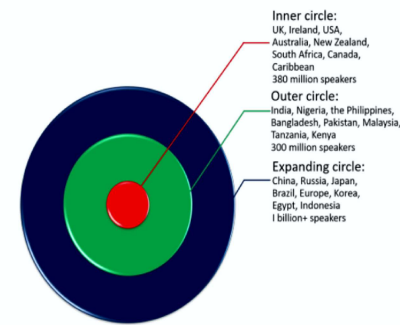

(Available from: https://www.eltconcourse.com/training/common/variety.html Accessed on July 5 , 2023)
The hard cell
Thanks to politics, stem cell research in the United States is suffering. But not so in Sweden, which is poised to capture what could be the biggest new market to hit biotech in a decade.
By Stephan Herrera
February 13, 2003
New York, January 1, 2006:
Sweden announces that one of its biotechnology companies is the first in the world to enter clinical trials with a new drug that could cure Alzheimer's disease. Four years ago this type of research was all but stopped in the United States by political and ethical questions − which is ...61... Sweden now seems in the best position to capture a $25 billion market.
Any day now, the U.S. Congress is expected to pass a sweeping new law that could dramatically inhibit researchers from working with stem cells taken from human embryos. Such cells, which can be used to grow a whole host of new cells and organs, could fundamentally change the way we treat heretofore intractable maladies like Alzheimer's disease, Parkinson's disease, cancer, stroke, liver failure, and heart disease. The only problem is that these cells by definition are derived from human embryos, many of which are cloned or come from unused fetuses collected at fertility clinics. The argument, from a certain segment of the American political spectrum, is that ...62... methods are morally wrong. They are ...63... a form of abortion or an activity that could eventually lead to human cloning.
Those working in stem cell research say the short-term effect of the legislation will be to further chill all forms of scientific inquiry and commercialization efforts in the field. Entrepreneurs and investors are already eschewing such research − in large part because of the additional uncertainty and risk that politics introduce.
Of the nearly 50 private stem cell companies in the United States, only a handful are still viable. Meanwhile, across the Atlantic, Sweden has avoided many of the political and ethical quagmires surrounding this type of research. It currently has 40 private stem cell companies, a number that's growing. Sweden's leading research universities have 32 percent of the world's stem cell inventory, close on the heels of the United States' 35 percent.
Sweden, say analysts, is now in the best position to
capture a worldwide market for drugs based on stem cell
therapies that could grow to $25 billion in the next three to five
years − nearly equal to the whole biotech industry at present.
This estimate doesn't even address the market for stem cells
capable of repairing damaged vital organs like the brain, heart,
and kidneys. If the United States offers an object lesson of what
can happen when scientific inquiry and investment capital fall victim to politics, Sweden and its leading stem cell startup,
NeuroNova, offer the opposite example. How odd that the
United States, which for generations has been the envy of the
world for its progressive views of science and commercialization,
should now have a biomedical climate chillier than a Swedish
winter.
One company feeling a lot of pain is StemCells, which at first glance seems to have it all: founding scientists include Stanford's Dr. Weissman and Fred Gage of the Salk Institute in La Jolla, California. An equally well-regarded expert in the treatment of Alzheimer's, Dr. Gage spent five years in Sweden as a researcher and now sits on a national committee on stem cell research there. The firm's chairman is Roger Perlmutter, Amgen's head of research.
Yet over the past two years, none of management's efforts to help investors and even critics reconsider the stem cell field have worked. At press time, the stock was thinly traded and sitting in the neighborhood of 50 cents. With less than $15 million in cash, the company likely won't exist at this time next year. (CEO Martin McGlynn, who joined the firm in January 2001, would not talk to Red Herring, despite repeated efforts.)
Some observers on Wall Street are asking, If StemCells can't make it, who can? Geron, the only other publicly held stem cell firm to speak of, is in a fix, too. The company's stock price is also moribund, at $3.85 per share. Thanks to some capital infusions a few years ago, when money came easy, Geron still has $40 million on hand, but by the end of next year, that too will likely be gone. Once a media darling, Geron focuses on diagnostic tests and drugs derived from stem cells, a strategy that's not going well. For the nine months ended last September, revenue fell 68 percent to $955,000 and net loss widened 18 percent to $26.7 million. The company's financials were also hit hard after it terminated an agreement with Pharmacia and acquired research technology from Lynx Therapeutics, which Geron bought in a desperate attempt to be seen as something more than just a stem cell company.
The situation is quite different, however, for Sweden's NeuroNova, which has 30 academic partners and a staff of 20. NeuroNova is working on ways to inject stem cells into the human brain to trigger a process called neurogenesis (the growth of new neural cells), which could combat diseases like Parkinson's, Alzheimer's, and even schizophrenia.
If NeuroNova is the first to develop a drug capable of
treating one of several central nervous system disorders − by far
the most lucrative after heart disease products − it will have
done so not because it raised more money or got more media
buzz than the rest. It will have succeeded because the science
is solid, and academe, government, and the investment
community are supportive. Meanwhile, the United States will
look on with envy and wonder how it, a country known for its
entrepreneurial innovation, ever got so short-sighted.
(Adapted from
http://www.redherring.com/investor/2003/02/biotech021303.html)
The hard cell
Thanks to politics, stem cell research in the United States is suffering. But not so in Sweden, which is poised to capture what could be the biggest new market to hit biotech in a decade.
By Stephan Herrera
February 13, 2003
New York, January 1, 2006:
Sweden announces that one of its biotechnology companies is the first in the world to enter clinical trials with a new drug that could cure Alzheimer's disease. Four years ago this type of research was all but stopped in the United States by political and ethical questions − which is ...61... Sweden now seems in the best position to capture a $25 billion market.
Any day now, the U.S. Congress is expected to pass a sweeping new law that could dramatically inhibit researchers from working with stem cells taken from human embryos. Such cells, which can be used to grow a whole host of new cells and organs, could fundamentally change the way we treat heretofore intractable maladies like Alzheimer's disease, Parkinson's disease, cancer, stroke, liver failure, and heart disease. The only problem is that these cells by definition are derived from human embryos, many of which are cloned or come from unused fetuses collected at fertility clinics. The argument, from a certain segment of the American political spectrum, is that ...62... methods are morally wrong. They are ...63... a form of abortion or an activity that could eventually lead to human cloning.
Those working in stem cell research say the short-term effect of the legislation will be to further chill all forms of scientific inquiry and commercialization efforts in the field. Entrepreneurs and investors are already eschewing such research − in large part because of the additional uncertainty and risk that politics introduce.
Of the nearly 50 private stem cell companies in the United States, only a handful are still viable. Meanwhile, across the Atlantic, Sweden has avoided many of the political and ethical quagmires surrounding this type of research. It currently has 40 private stem cell companies, a number that's growing. Sweden's leading research universities have 32 percent of the world's stem cell inventory, close on the heels of the United States' 35 percent.
Sweden, say analysts, is now in the best position to
capture a worldwide market for drugs based on stem cell
therapies that could grow to $25 billion in the next three to five
years − nearly equal to the whole biotech industry at present.
This estimate doesn't even address the market for stem cells
capable of repairing damaged vital organs like the brain, heart,
and kidneys. If the United States offers an object lesson of what
can happen when scientific inquiry and investment capital fall victim to politics, Sweden and its leading stem cell startup,
NeuroNova, offer the opposite example. How odd that the
United States, which for generations has been the envy of the
world for its progressive views of science and commercialization,
should now have a biomedical climate chillier than a Swedish
winter.
One company feeling a lot of pain is StemCells, which at first glance seems to have it all: founding scientists include Stanford's Dr. Weissman and Fred Gage of the Salk Institute in La Jolla, California. An equally well-regarded expert in the treatment of Alzheimer's, Dr. Gage spent five years in Sweden as a researcher and now sits on a national committee on stem cell research there. The firm's chairman is Roger Perlmutter, Amgen's head of research.
Yet over the past two years, none of management's efforts to help investors and even critics reconsider the stem cell field have worked. At press time, the stock was thinly traded and sitting in the neighborhood of 50 cents. With less than $15 million in cash, the company likely won't exist at this time next year. (CEO Martin McGlynn, who joined the firm in January 2001, would not talk to Red Herring, despite repeated efforts.)
Some observers on Wall Street are asking, If StemCells can't make it, who can? Geron, the only other publicly held stem cell firm to speak of, is in a fix, too. The company's stock price is also moribund, at $3.85 per share. Thanks to some capital infusions a few years ago, when money came easy, Geron still has $40 million on hand, but by the end of next year, that too will likely be gone. Once a media darling, Geron focuses on diagnostic tests and drugs derived from stem cells, a strategy that's not going well. For the nine months ended last September, revenue fell 68 percent to $955,000 and net loss widened 18 percent to $26.7 million. The company's financials were also hit hard after it terminated an agreement with Pharmacia and acquired research technology from Lynx Therapeutics, which Geron bought in a desperate attempt to be seen as something more than just a stem cell company.
The situation is quite different, however, for Sweden's NeuroNova, which has 30 academic partners and a staff of 20. NeuroNova is working on ways to inject stem cells into the human brain to trigger a process called neurogenesis (the growth of new neural cells), which could combat diseases like Parkinson's, Alzheimer's, and even schizophrenia.
If NeuroNova is the first to develop a drug capable of
treating one of several central nervous system disorders − by far
the most lucrative after heart disease products − it will have
done so not because it raised more money or got more media
buzz than the rest. It will have succeeded because the science
is solid, and academe, government, and the investment
community are supportive. Meanwhile, the United States will
look on with envy and wonder how it, a country known for its
entrepreneurial innovation, ever got so short-sighted.
(Adapted from
http://www.redherring.com/investor/2003/02/biotech021303.html)

Despite the potential for conflict, both Mr Bush and Mr Obama have stressed their willingness to work together in a bipartisan fashion during the transition phase since the latter beat John McCain, the Republican candidate, in last week’s election.


Fill out the gaps below with one the following words: that / where / which / who.
1. That’s the store ___ they buy their shoes.
2. The book, ___ we’ve been reading at school, was written long ago.
3. These are the directors and movies ___ I like.
4. Marie Curie is the woman ___ discovered radium.
Mark the alternative that fills out, correctly and respectively, the gaps in the sentences above.